Abstract
Singapore is the first Southeast Asian country to incorporate the routine use of Daratumumab in the treatment of newly diagnosed (in both transplant eligible and ineligible patients) and relapsed/refractory Multiple Myeloma patients. As real-world data amongst those with severe renal impairment and the Asian population are lacking, we present this nationwide retrospective study of Multiple Myeloma patients treated with Daratumumab outside of clinical trials from three major teaching hospitals. Data cut off was 31st December 2020. The median duration of follow-up was 10.4 months (range 0.3 to 45.7).
101 patients were recruited. 61.4% aged <65 years, 31.7% aged 65-74 years, and 6.9% aged ≥75. 57 patients had renal impairment defined by a creatinine clearance (CrCl) of <60ml/min; and 26 (45.6%) of them had CrCl <30ml/min, an exclusion criterion commonly applied in clinical trials. 47.5% of patients were ISS Stage III and 42.6% patients had high risk cytogenetics (denoted by the presence of del(17p), t(4;14), t(14;16) or aberrations of chromosome 1 by FISH).
32.7% patients received a prior autologous stem cell transplant. 77% patients were previously exposed to a proteosome inhibitor, 57% to an immunomodulatory drug, and 56% patients to both. Of the 76 patients who had ≥1 prior lines of therapy, 46 patients (60.5%) were refractory to the last line of therapy with 22.2% double-refractory. The median line of prior therapy was 1 (no prior lines (N=25), one prior line (N=40), two prior lines (N=22), three prior lines (N=12), four prior lines (N=2)). 89 patients (88.1%) received Daratumumab combination therapy (commonest: DRD (N=38), DVD (N=23), DPD (N=6) and DKD (N=6)).
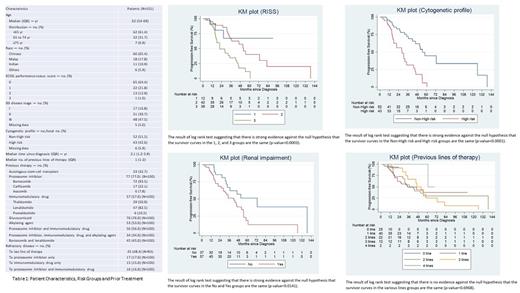
The progression free survival (PFS) was significantly better for lower RISS stages (vs higher), non-high risk (vs high-risk) genetic profile, and in the absence of renal impairment (p-value = 0.0003, <0.0001, and 0.0141 respectively by log-rank test). Interestingly, there was no difference in PFS by lines of prior therapy (0 to 4 lines) (p-value = 0.6968). This may be partly explained by the small number of heavily pre-treated patients in our cohort, reflecting real world clinicians' earlier positioning of Daratumumab amongst the available therapies.
The overall response rate (ORR) amongst all patients was 85.1% (30.7% ≥CR, 61.4% ≥VGPR). Amongst the 25 newly diagnosed myeloma patients, ORR was 92%, 36% ≥CR and 80% ≥VGPR. Amongst the 14 newly diagnosed patients with non-high-risk disease, the ORR was 85.7%; 14.3% ≥CR and 64.3% ≥VGPR. Amongst the 10 newly diagnosed patients with high-risk disease, ORR was 100%; 70% achieved ≥CR, 100% ≥VGPR.
40 patients received 1 prior line of therapy with ORR 90%; ≥CR 40% and ≥VGPR 62.5%. Of the 19 non-high-risk patients with 1 prior line of therapy, the ORR was 89.5%, 47.4% ≥CR and 68.4% ≥VGPR. Amongst the 18 high-risk patients with 1 prior line of therapy, the ORR was 88.9%; 38.9% ≥CR and 55.6% ≥VGPR. 36 patients received ≥2 prior lines of therapy: their ORR was 61.1%; 16.7% ≥CR and 47.2% ≥VGPR. 19 of the 36 patients with non-high-risk disease had an ORR of 73.7%, 10.5% ≥CR and 57.9% ≥VGPR. 15 of the 36 patients with high-risk disease had an ORR of 46.7%; 20% ≥CR and 33.3% ≥VGPR.
24.8% patients experienced an infusion adverse reaction (AE) of any grade, 19 during the first infusion and 6 during both first and second infusions. 2 patients experienced grade ≥3 reactions. 13 (12.9%) patients experienced hematological AEs; 3 cases were grade ≥3. 33 (32.7%) patients experienced non-hematological AEs, 3 were grade ≥3 (commonest being pneumonia). There was no AE-related mortality. Temporary treatment interruption was noted in 16 patients; but permanent discontinuation occurred in only 1 patient. Our AE incidences, especially infusion AEs were significantly lower than clinical trial studies. 13 of 16 patients previously exposed to hepatitis B were given entecavir prophylaxis. No reactivation was noted in the entire cohort.
Of the 36 patients who experienced relapse after Daratumumab, 5 (13.9%) had discordance between biochemical/histological findings and clinical relapse; and 14 (38.9%) relapsed with extramedullary and subcutaneous plasmacytomas, malignant pleural effusions and plasma cell leukemia.
Daratumumab seems to produce similar response rates in high risk versus non-high-risk patients; amongst the newly diagnosed and those at first relapse; in an Asian cohort with higher risk disease and severe renal impairment.
Disclosures
Tso:J&J: Honoraria; GSK: Honoraria; Amgen: Honoraria. Chng:Amgen: Honoraria; J&J: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; BMS: Honoraria; Abbvie: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Hummingbird: Research Funding. Goh:Roche: Honoraria; Medical Pharma: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; NS Pharma: Honoraria; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Johnson & Johnson: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Antengene: Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria; Astellas: Honoraria. Ooi:BMS: Honoraria; Abbvie: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Astellas: Honoraria; Amgen: Honoraria; Pfizer: Honoraria. Chen:Novartis: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Takeda: Honoraria. Nagarajan:Janssen: Honoraria, Research Funding, Speakers Bureau; Amgen: Honoraria, Research Funding, Speakers Bureau; BMS/Celgene: Honoraria; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Tan:Johnson & Johnson: Honoraria; BMS: Honoraria; Roche: Honoraria; Astra Zeneca: Honoraria. Ong:Novartis: Honoraria; Janssen: Honoraria; Antengene: Honoraria; Astra Zeneca: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal